We keep our
finger on the
pulse of the
health industry.
Dec 03, 2024
Novateur, BIO, Guide to JPM, app
 ‘22
‘22
Jan 07, 2022
COVID-19, Omicron, Vaccine, Pandemic

Nov 22, 2021
COVID-19, Vaccines, Coronavirus, SARS-CoV-19, Nature

Sep 23, 2021
Covid-19, PoC, Diagnostics

May 07, 2021
diabetic macular edema, vision impairment, diabetes mellitus, diagnosis, ophthalmology, ophthalmic pearls

Mar 05, 2021
COVID-19; SARS-CoV-2; vaccine; target product profile; immune response; coronavirus; clinical trial; public health
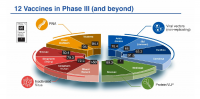
Feb 12, 2021
Business, Biotech, Life Sciences, Investment, British Columbia, Venture Capital, COVID-19
 ‘21
‘21
Jan 12, 2021
COVID-19; point-of-care diagnostic test, target product profile, clinical performance

Dec 10, 2020
COVID-19, SARS-CoV-2, Virtual Teams, Global Teams

Aug 06, 2020
COVID-19, SARS-CoV-2, leukotrienes, cytokine storm, coronavirus, inflammatory response, vascular leak, clinical trial

Jul 04, 2020
COVID-19, SARS-CoV-2, diagnostics, serological diagnostic test, performance
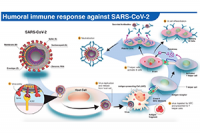
Jun 19, 2020
COVID-19, SARS-CoV-2, vaccine, immune response, coronavirus, clinical trial, public health
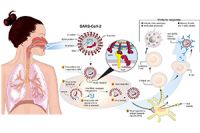
Jun 11, 2020
COVID-19, novel coronavirus, coronavirus, SARS-CoV-2, diagnostics, diagnostic tests, Food and Drug Administration, FDA, European Union, EMA, Health Canada
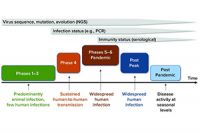
May 27, 2020
COVID-19, coronavirus, vaccine, strategy, vaccine manufacturing, drug development, Canada, Health Canada

May 04, 2020
Regulatory, FDA, Emergency Use Authorization, EUA, COVID-19, SARS-CoV-2, serological diagnostics regulatory pathway, diagnostics, antibody tests
 ‘20
‘20
Apr 28, 2020
Regulatory, FDA, Emergency Use Authorization, EUA, COVID-19, SARS-CoV-2, Diagnostics, Devices
Nov 19, 2019
Cannabinoid Biosynthesis Landscape, Cannabis

Mar 25, 2019
Health Science, Leadership, Science, Technology, Women in Science
 ‘19
‘19
Mar 22, 2019
Entrepreneurship, Financing, Investing, Venture Capital

Oct 23, 2018
Biotechnology, Drug Development, Events, Life Sciences, Novateur, Pharmaceutical

Oct 03, 2018
Biotechnology, Canada, Events, Financing, Life Sciences, Novateur, Pharmaceutical

Sep 07, 2018
Biotechnology, Life Sciences, Novateur, Pharmaceutical

Sep 04, 2018
Clinical Development, EMA, Regulatory
 ‘18
‘18
Feb 26, 2018
Biotechnology, Cancer Research, Fundraising, Novateur

Nov 28, 2017
Novateur, Regulatory

Nov 23, 2017
Cannabis, Health Canada

Nov 07, 2017
Biotechnology, Companion Diagnostics, Events

Nov 07, 2017
Biotechnology, British Columbia, Government, Life Sciences

Oct 10, 2017
Events, Financing, Novateur

Oct 10, 2017
Biotechnology, Companion Diagnostics, Drug Development, Pharmaceutical
Sep 26, 2017
Career, CMC, Drug Development, Novateur

May 11, 2017
505(b)(2), Drug Development, Label, NDA, Pharmaceutical, Regulatory
 ‘17
‘17
Apr 10, 2017
Biotechnology, Drug Development, Pharmaceutical

Nov 21, 2016
FDA, Government, Medical Devices, Pharmaceutical, Regulatory

Nov 21, 2016
Drug Development, FDA, Government, Pharmaceutical, Regulatory

Nov 03, 2016
Biotechnology, Drug Development, FDA, Pharmaceutical

Sep 07, 2016
505(b)(2), FDA, NDA

May 26, 2016
Biotechnology, Drug Development, Pharmaceutical

Apr 14, 2016
Biotechnology, Drug Development, Financing, Pharmaceutical

Feb 18, 2016
Biotechnology, Public Markets
 ‘16
‘16
Feb 11, 2016
Government, Market Access, Pharmaceutical, Research

Dec 10, 2015
Drug Development, EMA, FDA, Regulatory

Dec 03, 2015
approval, drug development, drug label, efficacy, FDA, IND, label, pre-IND, regulatory, safety, submission

Nov 13, 2015
claims, intellectual property, legal, Nautilus, patents

Nov 03, 2015
biotechnology, diagnostics, drug development, EMA, European Medicines Agency, FDA, global environment, Health Canada, medical devices, MHLW, pharmaceutical, regulatory

Oct 21, 2015
Accelerated Approval Pathway, Breakthrough Therapy Designation, drug development, expedited development, expedited programs, Fast Track Designation, FDA, Priority Review Designation, regulatory, regulatory authorities, review programs

Oct 09, 2015
biotech, financing, biotechnology, drug pricing, financing, pharma, pharmaceutical, public markets, stock exchange

Jul 09, 2015
certified reference standard, CMC, development, R&D, reference standard, research

Jun 25, 2015
biotechnology, development, EMA, EU, European Medicines Agency, European Union, pharma, regulatory

Jun 17, 2015
biotechnology, global environment, pharma, virtual operations, virtual team

Jun 11, 2015
biotechnology, clinical development, clinical trial, pharma, quality, regulatory development

Jun 04, 2015
biotechnology, clinical development, regulatory development, virtual operations, virtual team
 ‘15
‘15
May 27, 2015
biotechnology, clinical development, regulatory development, virtual operations, virtual team

We keep our
finger on the
pulse of the
health industry.
Subscribe to our newsletter for access to latest updates.
Access Novateur news as well as FDA and Expert Databases.
Check out conferences and events we are attending!
test
