We keep our
finger on the
pulse of the
health industry.
Our experts are publishing articles and supporting companies that are working with health solutions to help solve the global pandemic.
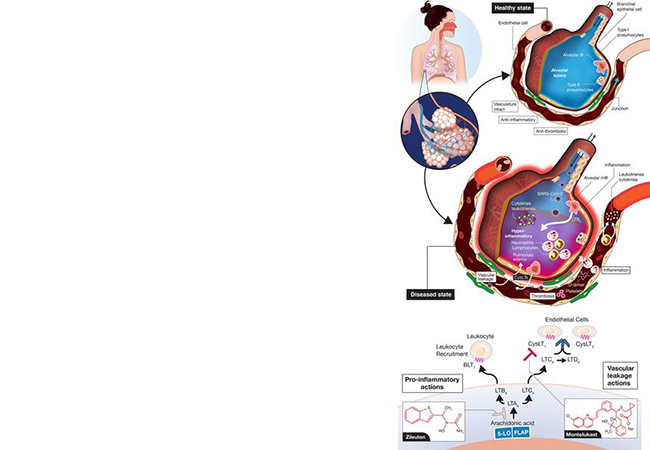
Published in major journals and media, the following features are tracking the evolving developments of the COVID-19 pandemic.











Subscribe to our newsletter for access to latest updates.
Access Novateur news as well as FDA and Expert Databases.
Check out conferences and events we are attending!